Risks you will not assume If you hire a CRO
What is a CRO, how do they provide support to the pharmaceuticals, and which are the advantages of outsourcing clinical trials management? Keep reading!
Those who are already familiar with CROs may not need a clarification of what these initials mean, but we prefer to start our post with what we think should be the beginning. CRO is the acronym of Contract Research Organization and refers to companies that, for several decades, have been a business model that provides support and assistance to the pharmaceutical industry.
What is a CRO?
CROs are organizations that aid through the management and development of clinical trials to sponsors of clinical research (pharmaceutical industry, biotechnology, and medical devices). With their specialization, experience, and knowledge in this niche, they ensure that the trials are carried out in accordance with the ethical and methodological standards for the compliance with current regulations and quality standards.
This specialization is a guarantee of the veracity, reliability and traceability of the results obtained, essential for the registration and commercialization of a vaccine or any other treatment.
The sponsors outsource several activities to the CROs to carry out and supervise clinical research projects so that:
They guarantee quality and compliance with the regulations in force during the development of the research project.
Analyze the data collected during the research process with advanced techniques and present the final results.
Give assistance throughout the project or in some specific phases.
Project management in clinical trials is a complex task that requires a very strict organization to ensure that all phases are being executed as stablished in the protocol. Many actors are involved: manufacturers, sponsors, CROs, ethics committees, Regulatory authorities, Researchers, research centers, … and they always must work under the established quality standards that ensure the validity of the study.
CROs are a support in this task, since they are in charge of the integral management of the project and act as interlocutors among all organizations or people involved.
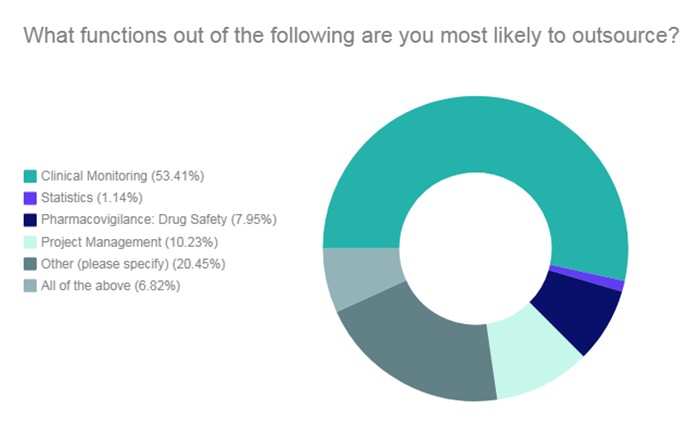
A study carried out by PharmaIQ, the tasks that are most often outsourced to CROs are (ordered from highest to lowest score): Monitoring, “others”, project management, pharmacovigilance, all of them, and statistical services.
Compliance with ISO standards and Good Clinical Practices (GCP-ICH Guidelines)
Every trial (clinical, nutritional, …) must be performed in compliance with the GCP (Good Clinical Practices) from the VICH program that ensure reliable data and the protection of the health of living beings. Also, It is fundamental the implementation of ISO standards that lay the foundations of quality management.
CROs ensure that all clinical trial requirements are met
- Correct protocol design
- Rigorous and Ethical Method
- Compliance with the ethical and legal standard
- Veracity of the Information
- Information Traceability
- Information quality
- Dissemination of Results
Advantages of outsourcing the trials management to a CRO
By outsourcing the management of these services, pharmaceutical companies obtain many advantages that vary depending on studies characteristics. Still, we could highlight the following ones:
CROs have innovative technological tools (due to their own specialization that makes them be up to date and improve their strategies to maintain competitiveness and improve the quality of their services). It increases the efficiency of the study processes.
Minimization of economic investment and cost reduction.
Less risks assumed
More detail of the Pharmacovigilance (or product safety) activities.
By acting as an interlocutor throughout the trial, they ensure effective communication and the correct development of the study. In addition, as they are entirely involved in these activities, all their efforts are focused on continuous improvement.
They have a multidisciplinary team trained and qualified in various areas, providing specialized human resources.
Compliance with times.
Clinical trials monitoring
Monitoring activities have a huge importance as they ensure the safety of animals (or patients in case of human research), the veracity, reliability and traceability of the data, and the quality of the research.
It is carried out a “training” at the beginning of each study (before its start) on: Protocol, GCP, responsibilities of each investigator, study logistic, data collection, documents, dates, files,…
It is fundamental that each investigator and professional that takes part in the study knows and understand the characteristics of the entire project in order to avoid fatal data errors.
Periodic visits to research centers: These visits aim to ensure compliance with ethical standards, verify the correct inclusion of the animals subject to study and the data recorded in the documents, … This allows correcting errors or inconsistencies in the data and check if Adverse Events have occurred (and if they have been notified as established in the protocol)…
Monitorization is also a guarantee of data quality: It is fundamental continuous communication with the investigator responsible of cleaning, validating, and analyzing the data. This facilitates the efficient resolution of inconsistencies and errors, as well as that a correct record of “who does what”.
All these activities, (we have said them very briefly and highlighting the main points), seem quick or easy to do. If so, the concept of monitoring would not exist since all this guidelines would be assumed to be met.
Types of CROs
We can distinguish the types of CROs basing on two factors.
They could offer either assistance throughout the project (full-service CRO) or only in specific phases. Outsourcing the entire project to a unique organization is more recommendable for obvious reasons (as they have a better understanding and global vision, they can do a more exact and precise work). Still, this decisions are influenced by numerous reasons such as research objectives, technical aspects, and sponsors resources.
On the other hand, we can classify them in terms of market coverage: global or local companies. Global companies have a greater coverage but have less flexibility while the local ones can adapt to clients’ requirements more easily (although they have less notoriety).
What should I consider when selecting a CRO to perform trials?
To start with, the decision will depend on study purposes, technical aspects, and budget. Also, it is a plus for many pharmaceutical when a CRO can offer personalized assistance in all phases involved in the project (sample size, protocol design, monitorization…) before it starts.
All this, with the maximum transparency in their activities and costs, and a proactive attitude for solving problems.
Going back to the study that we mentioned before from PharmaIQ, most of the companies that participated in it affirmed that the quality of the services is the most important criterion when selecting (almost 60%). Second, the study highlighted their reputation and experience in the market as important factors to consider.
About our CRO – clinical and nutritional trials with animals
At this point, we hope we have clarified the main responsibilities of these companies and they role in the industry. We have been more than 20 years performing clinical and nutritional trials in animals and our slogan “accuracy and precision” resumes our know-how.
Our aim is to guarantee food safety by performing clinical and nutritional trials with animals. We verify the safety, efficacy and quality of veterinary products.
We offer services in either all phases involved in the trials (from protocol design to the final report) or in specific phases. We adapt to our clients, to whom we always appreciate the trust placed in us and our professionalism.
T&T seeks continuous improvement in order to be up-to-date with the current regulations and innovative techniques.
Contact us for more information.
References
Henry, C. (2018, 27 marzo). Top 10 Clinical Research Organisations in the Pharma and Biotech Industry. Pharma IQ. https://www.pharma-iq.com/pre-clinical-discovery-and-development/articles/top-10-clinical-research-organisations-in-the